J. Marvin Herndon's Q&A About Earth's Composition |
Q: We have hardly scratched Earth's surface. How can we know its composition?
A: (1) The isotope compositions of the elements in Earth, Moon, meteorites and presumably all the all objects in the Solar System are essentially the same, meaning that the primordial matter of the Solar System is of common origin; (2) The elemental composition of that matter is still evident in the photosphere of the Sun and, for non-volatile elements, in certain meteorites called chondrites.
Q: So, Earth is like a chondrite meteorite. Is it as simple as that?
A: Yes, Earth is like a chondrite. But it is not simple, because there are three different types (or groups) of chondrites with similar bulk compositions, but which have very different mineral compositions. These are: Carbonaceous Chondrites, Enstatite Chondrites, and Ordinary Chondrites.
Q: What causes the chondrites to have different mineral compositions?
A: One element, oxygen, makes all the difference. In carbonaceous chondrites virtually every element is combined with oxygen. So, there is no iron metal, no iron alloy to make a massive-core planet like Earth.
Q: There is iron metal in ordinary chondrites and in enstatite chondrites, so which does Earth resemble?
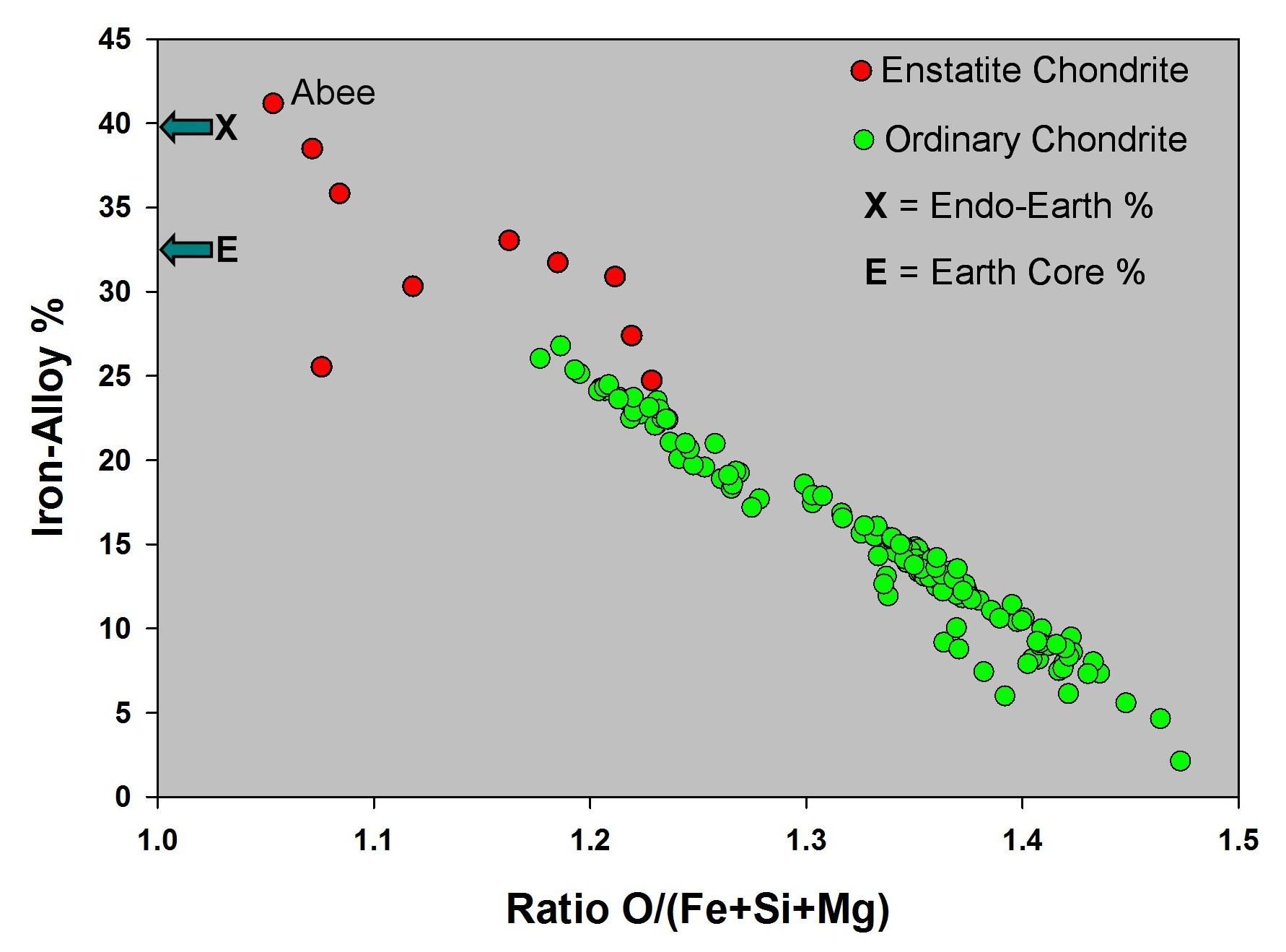 A: For about
seventy years, geoscientists have assumed that Earth resembles an
ordinary chondrite. Why? Because ordinary chondrites are the most common
meteorites observed falling to Earth, whereas enstatite chondrites are rare and
contain unusual minerals, e. g., CaS, not found on the Earth's surface. But that
is an incorrect assumption.
A: For about
seventy years, geoscientists have assumed that Earth resembles an
ordinary chondrite. Why? Because ordinary chondrites are the most common
meteorites observed falling to Earth, whereas enstatite chondrites are rare and
contain unusual minerals, e. g., CaS, not found on the Earth's surface. But that
is an incorrect assumption.
Q: Why is does Earth not resemble an ordinary chondrite but does resemble an enstatite chondrite?
A: The Earth's core comprises about 32% of the planet's mass. Only enstatite chondrites (red in figure at right), not ordinary chondrites (green in figure at right), have the necessary amount of iron alloy to comprise such a massive core. The ordinary chondrites are more oxygen-rich, so some of their iron occurs as FeO in the silicates.
Q: Are there any other reasons why Earth resembles an enstatite chondrite?
A: Yes. As discovered by J. Marvin Herndon,
the mineralogically determined parts of the Abee enstatite chondrite,
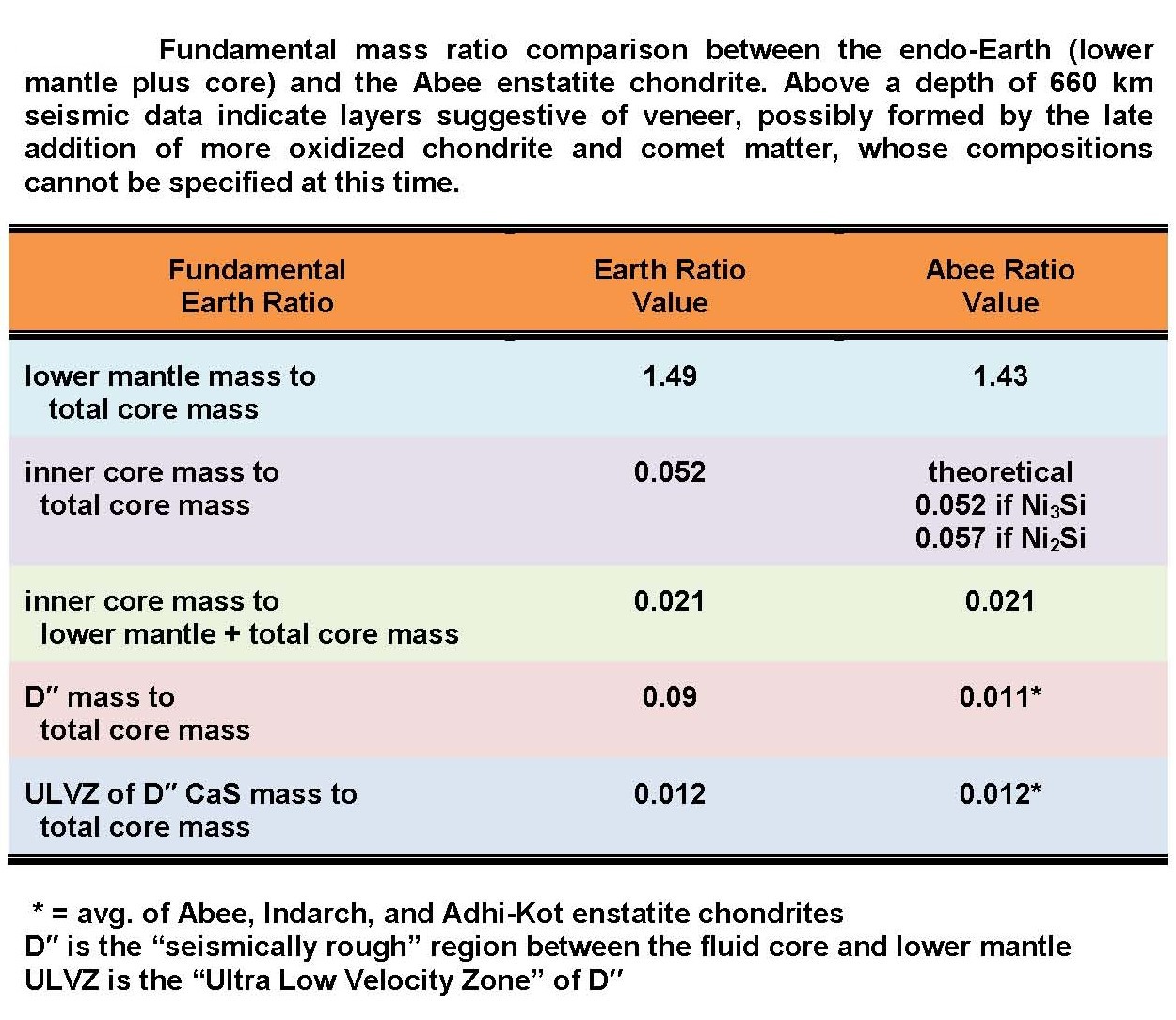 expressed
as mass ratios, match quite precisely corresponding seismically
determined parts of the Earth as shown in the table at right.
expressed
as mass ratios, match quite precisely corresponding seismically
determined parts of the Earth as shown in the table at right.
Q: So, in easy to understand terms, what does Earth resembling an enstatite chondrite mean?
A: First, it means that the inner 82% of the Earth has the same oxygen-starved state of oxidation as the matter from which certain enstatite chondrites formed.
Q: How is that oxygen-starved oxidation state different than an ordinary chondrite oxidation state?
A: The inner 82% of Earth formed under circumstances that limited available oxygen, Consequently, some of the oxygen-loving elements could not form oxides and ended up in the iron alloy of the core. For example, some silicon, some magnesium, some calcium, and much uranium ended up in the core. If instead, Earth were to have resembled an ordinary chondrite, those elements would have ended up as oxides in the silicate mantle.
Q: What formation circumstances caused the matter of the inner 82% of Earth to be oxygen-starved?
A: Thermodynamic calculations by Arnold Eucken and by J. Marvin Herndon and Hans E. Suess indicate that condensing, i.e., "raining out", from a gas of solar composition at high pressures (100 to 1000 times our atmospheric pressure) and high temperatures (perhaps 2500 degrees C. or ~4500 degrees F.), would lead to such an oxygen-starved condensate. Such circumstances are expected to occur in the center of a giant gaseous protoplanet.
Q: But are not those temperatures above the melting point of iron metal?
A: Yes. And, under those circumstances
molten iron, with the elements that dissolve in it, would be the most refractory
condensate, the first substance to condense. In
other words, the Earth's core
would form before it's silicate mantle.
Q: Suppose that the enstatite-chondrite-like Earth's core has fully condensed and is completely molten. What happens as it starts to cool?
A: The figure at right shows the major and minor elements of the Earth's core and lower mantle by analogy with the Abee meteorite. Note that small amounts of oxygen-loving calcium, magnesium, and silicon occur in the core. Much of the oxygen-loving trace element uranium also occurs in the core. As the core starts to cool, these oxygen-loving elements, which are incompatible in the iron alloy, find thermodynamically feasible ways to "escape" by combining with another element and precipitating out of solution.
Q: So, what compounds precipitate out and where do they go?
A: First, consider the elements that form
high-melting point compounds with sulfur. Calcium and magnesium sulfide (CaS and
MgS) will precipitate and float to the top of
 the core because they are less
dense than the iron-sulfur liquid core. Uranium, presumably as the sulfide, US,
will sink to Earth's center because it is the most dense substance.
the core because they are less
dense than the iron-sulfur liquid core. Uranium, presumably as the sulfide, US,
will sink to Earth's center because it is the most dense substance.
Q: On what basis can it be said that calcium and magnesium will react with sulfur in that manner?
A: There is an industrial process to remove traces of sulfur from high-grade steel. Calcium or magnesium is injected into the molten steel where it forms those sulfides which float to the surface.
Q: But what about the effect of pressures deep inside Earth?
A: The high-pressures will not change the chemistry of these simple compounds.
Q: So, after the high-temperature sulfides precipitate, what happens next?
A: Upon further cooling the silicon will combine with nickel as the compound nickel silicide, which sinks to form Earth's inner core.
Q: Playing the Devil's advocate, what if Earth does not resemble an enstatite chondrite but does instead resemble an ordinary chondrite; would there be Earth-core precipitates?
A: If the Earth were like an ordinary chondrite, which is not the case, there would be no high-temperature Earth-core precipitates because there would be no oxygen-loving elements in the core.
Q: What happens to the uranium accumulation, presumably as a sulfide, at the center of the Earth.
A: The uranium will function as a natural nuclear fission reactor, called the georeactor, that is the energy source and generator of the Earth's magnetic field and the energy supply for surface "hot-spots" such as underlies Hawaii and Iceland (click here). There are so many questions and answers about the georeactor that these comprise a Q&A webpage of their own (click here).
Q: So, after the core fully condenses then what happens?
A: The silicate of the lower mantle (MgSiO3, almost devoid of oxidized iron, FeO) condenses. Together, the core and the lower mantle comprise the Endo-Earth, the inner 82% of Earth that is bounded by the seismic discontinuity at a depth of about 660km.
Q: What is above the 660km seismic boundary.
A: Above the seismic discontinuity at 660km depth, there are more seismic discontinuities in the upper mantle. Rocks brought to the surface from the upper mantle contain oxidized iron, FeO. Some have a "chondritic complement" indication of the addition of carbonaceous and/or ordinary chondrite matter.
Q: What are the compositions of the layers of the upper mantle?
A: At present one cannot say without making arbitrary assumptions.
Q: Under the belief that Earth is like an ordinary chondrite, how have the various structures within the Earth been explained?
A: The Earth's mantle (upper plus lower) has been assumed to be of uniform chemical composition with the seismic discontinuities assumed to result from pressure-induced changes in crystal structure. The inner core has been assumed to be iron in the process of crystallizing from the fluid iron alloy core. These are all assumptions with no corroborating evidence.
Q: So, what happens next after the lower condenses and upper mantle condenses and forms?
A: Mantle condensation is followed by full condensation of about 300 Earth-masses of ices and gases to yield a fully condensed Jupiter-like gas giant planet.
Q: How were the gases removed?
A: Presumably, Earth's gas-giant envelope of gases and ices was stripped off by the superintense T-Tauri phase solar wind as the Sun started to burn, leaving behind a rocky planet that had been compressed by the weight of some 300 Earth-masses of overburden gases and ices. This and the consequences that follow are explained by J. Marvin Herndon's Whole-Earth Decompression Dynamics (click here).