
Deep-Earth Oxidation State
 
Deep-Earth Oxidation State |
Those meteorites that are called chondrites have elemental compositions quite similar to the elemental composition of the photosphere of the Sun (typically to within a factor two for rock-forming elements). For that and other reasons, Earth is thought to resemble a chondrite meteorite. But there is a complication: Chondrites fall into three groups distinguished by distinctly different mineral assemblages. So, these are the questions to be answered: How are they different? How did those differences come about? What does this say about the Earth's interior and its origin?
Carbonaceous Chondrites = These are oxygen-rich and relatively rare. Virtually all elements are combined with oxygen. Even sulfur is combined with oxygen. There is essentially no iron metal.
Ordinary Chondrites = These are less oxygen-rich and are common. Some of their iron is combined with oxygen in silicate minerals; the balance of their iron is as metal or iron sulfide. For more information on the origin of ordinary chondrites, click here.
Enstatite Chondrites = These are oxygen-poor and relatively rare. Virtually all of their iron is present as iron metal/sulfide which also contains a portion of oxygen-loving elements, such as Si, Mg and Ca.
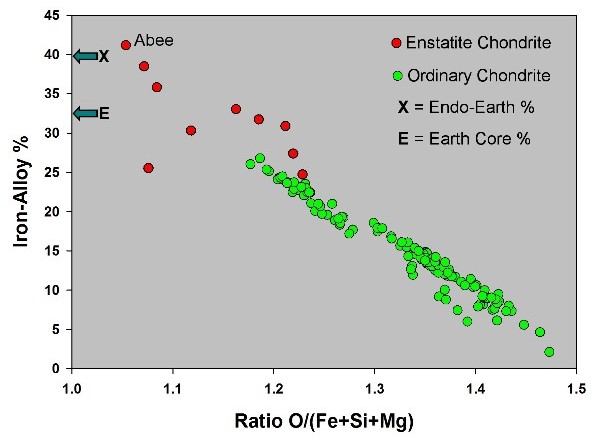 Only
ordinary chondrites and enstatite chondrites have significant amounts of iron
metal alloy. If heated to a high temperature, the iron sulfide melds with iron
metal forming a liquid alloy that is more dense than the silicate-rock portion.
The parallel with Earth's fluid core and solid rock-mantle is obvious. But does
Earth resemble an ordinary chondrite as believed since 1940 or an enstatite
chondrite as shown by J. Marvin Herndon in 1980? The figure at right shows that
only an enstatite chondrite, not an ordinary chondrite, has sufficient iron
alloy to resemble the Earth with its massive core.
Only
ordinary chondrites and enstatite chondrites have significant amounts of iron
metal alloy. If heated to a high temperature, the iron sulfide melds with iron
metal forming a liquid alloy that is more dense than the silicate-rock portion.
The parallel with Earth's fluid core and solid rock-mantle is obvious. But does
Earth resemble an ordinary chondrite as believed since 1940 or an enstatite
chondrite as shown by J. Marvin Herndon in 1980? The figure at right shows that
only an enstatite chondrite, not an ordinary chondrite, has sufficient iron
alloy to resemble the Earth with its massive core.
 Some
elements have a higher affinity for oxygen than others. Silicon (Si), magnesium
(Mg), and calcium (Ca), for example, have a higher affinity for oxygen than does
iron (Fe). In ordinary chondrites all of the high-oxygen-affinity elements are
combined with oxygen; some iron is also combined with oxygen in the silicates.
In primitive enstatite chondrites, such as Abee, iron combined with oxygen
essentially does not occur; even some elements that have a high affinity for
oxygen are not fully combined with oxygen. The oxidation state is referred to as
"highly reduced". In fact such meteorites represent the most highly reduced
mineral assemblage occurring in nature. How enstatite chondrite matter attained
that highly reduced oxidation state while forming from an atmosphere of solar
composition was a great mystery. In 1976, J. Marvin Herndon and Hans E. Suess
showed from thermodynamic considerations that solar-matter condensation at high
pressures and high temperatures would lead to highly-reduced condensate,
provided the condensate was isolated from further reactions at lower
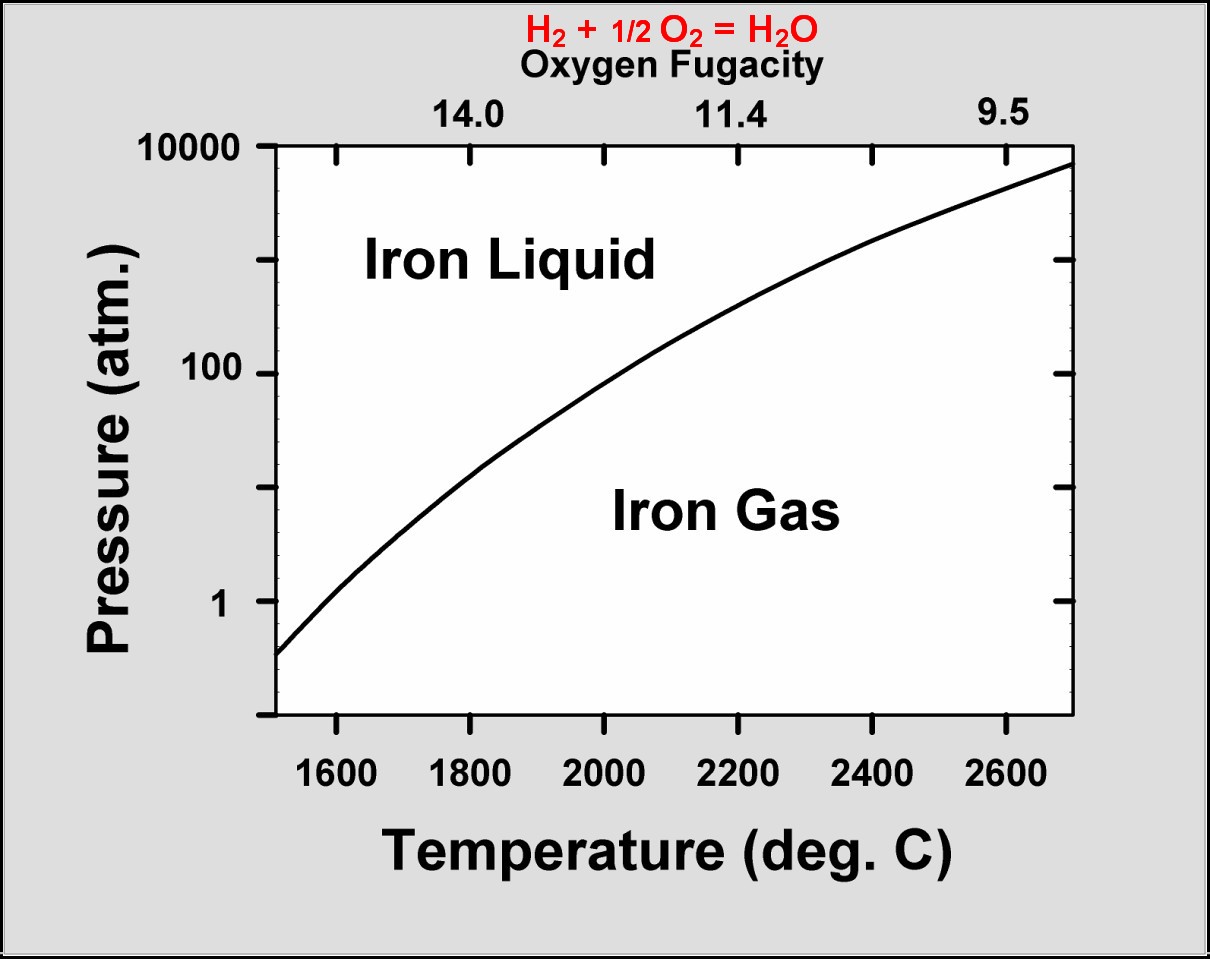
temperatures [1, 2]. This is why: Ideally, a substance can begin to condense
when the partial pressure of the substance in the gas exceeds the vapor pressure
of the condensed substance. So, at higher pressures, substances condense at
higher temperatures. The state of oxidation of the condensate, however, is
determined by the gas-phase reaction: H2+ŻO2=H2O
which is a function of temperature, but independent of pressure. High pressure
condensation occurs at high temperatures in a highly reducing environment (see
figure at left). Low-pressure condensation occurs at low temperatures in a much
more oxidizing environment.
Some
elements have a higher affinity for oxygen than others. Silicon (Si), magnesium
(Mg), and calcium (Ca), for example, have a higher affinity for oxygen than does
iron (Fe). In ordinary chondrites all of the high-oxygen-affinity elements are
combined with oxygen; some iron is also combined with oxygen in the silicates.
In primitive enstatite chondrites, such as Abee, iron combined with oxygen
essentially does not occur; even some elements that have a high affinity for
oxygen are not fully combined with oxygen. The oxidation state is referred to as
"highly reduced". In fact such meteorites represent the most highly reduced
mineral assemblage occurring in nature. How enstatite chondrite matter attained
that highly reduced oxidation state while forming from an atmosphere of solar
composition was a great mystery. In 1976, J. Marvin Herndon and Hans E. Suess
showed from thermodynamic considerations that solar-matter condensation at high
pressures and high temperatures would lead to highly-reduced condensate,
provided the condensate was isolated from further reactions at lower
temperatures [1, 2]. This is why: Ideally, a substance can begin to condense
when the partial pressure of the substance in the gas exceeds the vapor pressure
of the condensed substance. So, at higher pressures, substances condense at
higher temperatures. The state of oxidation of the condensate, however, is
determined by the gas-phase reaction: H2+ŻO2=H2O
which is a function of temperature, but independent of pressure. High pressure
condensation occurs at high temperatures in a highly reducing environment (see
figure at left). Low-pressure condensation occurs at low temperatures in a much
more oxidizing environment.
As discovered by J. Marvin Herndon, the parts of a primitive enstatite match quite precisely the parts of the Earth below a depth of 660km which means the deep-interior of Earth has an oxidation state virtually identical to that enstatite chondrite meteorite [3-10].
For more information, see the following WebPages:
Herndon's Nickel
Silicide Inner Core Composition (click here)
Herndon's
Composition of Earth's Interior Parts Below 660 km (click here)
Herndon's Composition of D'' (click here)
Answered Questions
about Earth's Interior Composition (click here)
| References | |
| 1. |
Herndon, J. M. and Suess, H. E., Can enstatite chondrites form from a nebula of solar composition. Geochimica et Cosmochimica Acta, 1976, 40, 395-399. |
| 2. | Herndon, J. M., Solar System processes underlying planetary formation, geodynamics, and the georeactor. Earth, Moon and Planets, 2006, 99, 53-99. (click here for pdf) |
| 3. | Herndon, J. M., The nickel silicide inner core of the Earth. Proceedings of the Royal Society of London, 1979, A368, 495-500. (click here for pdf) |
| 4. | Herndon, J. M., The chemical composition of the interior shells of the Earth. Proceedings of the Royal Society of London, 1980, A372, 149-154. (click here for pdf) |
| 5. | Herndon, J. M., The object at the centre of the Earth. Naturwissenschaften, 1982, 69, 34-37. |
| 6. | Herndon, J. M., Feasibility of a nuclear fission reactor at the center of the Earth as the energy source for the geomagnetic field. Journal of Geomagnetism and Geoelectricity, 1993, 45, 423-437. (click here for pdf) |
| 7. | Herndon, J. M., Sub-structure of the inner core of the Earth. Proceedings of the National Academy of Sciences USA, 1996, 93, 646-648. (click here for pdf) |
| 8. | Herndon, J. M., Composition of the deep interior of the Earth: Divergent geophysical development with fundamentally different geophysical implications. Physics of the Earth and Planetary Interiors, 1998, 105, 1-4. |
| 9. | Herndon, J. M., Scientific basis of knowledge on Earth's composition. Current Science, 2005, 88, 1034-1037. (click here for pdf) |
| 10. | Herndon, J. M., Geodynamic basis of heat transport in the Earth. Current Science, 2011,101, 1440-1450. (click here for pdf) |